Methods
- About COPSAC
- Research team
- Hans Bisgaard
- Mette Damgaard Nielsen
- Katrine Grau
- Birgit Nielsen
- Marianne Mikkelsen
- Ulrik Ralfkiaer
- Connie Albinski
- Dorte Andersen
- Dion Aagaard-Hansen
- Michael Westenholz
- Brian Vegas
- Alma Pedersen
- Bo Chawes
- Jakob Stokholm
- Nadja Vissing
- Klaus Bønnelykke
- Johannes Waage
- Tarun Ahluwalia
- Anna Thysen
- Morten Rasmussen
- Johan de Rooi
- Leon Jessen
- Helene Wolsk
- Henrik Hallas
- Tine Pedersen
- Rebecca Vinding
- Sunna Thorsteinsdottir
- Astrid Sevelsted
- Lambang Arianto
- Jonathan Thorsen
- Nadia Fink
- Asja Kunøe
- Sarah Pedersen
- Mathias Hansen
- Sine Rossen
- Pia Nørrisgaard
- Louise Monnerup
- Pernille Fjordholt
- Lena Vind
- Hanne Nissen
- Britta Hansen
- Simone Hansen
- Ann-Marie Schoos
- Kristoffer Linder
- Michael Lofvall
- Martin Mortensen
- Ni Wang
- Christian Carlsson
- Malene Rosenberg
- Emil Christensen
- Mia Bjørg Olsen
- Hedda Løvenhøj
- Jens Heller Frederiksen
- Pernille Tegner Fjordholt
- Shiraz Shah
- Lærke Sass
- Mathis Hjelmsø
- Line Reinvard
- Nilo Følsgaard
- Daniela Rago
- Anders Eliasen
- Nicklas Brustad
- Line Reinvard
- Rebekka Søgaard
- Casper Pedersen
- Rebekka Søgaard
- Jenni Lehtimäki
- Nicklas Brustad
- Julie Rosenberg
- Gözde Gürdeniz
- Mads Nørregaard
- Caroline Kleis
- Laura Hesselberg
- Rikke Sunde
- Tamsin Redgwell
- Mathias Elsner Melgaard
- Signe Kjeldgaard Jensen
- Maibritt Clausen
- Sofie Ingwersen
- Matteo Soverini
- Marie-Louise Hjuler
- Mie Liljendahl
- Michelle Pedersen
- Sarah Brandt
- Julie Kyvsgaard
- David Horner
- Matthew Stevenson
- Amelie Fritz
- Luo Yang
- Jesper Rasch
- Frederikke Rosenvinge Skov
- Christina Poulsen
- Clara Vilhelmsen
- Benthe Vink
- Danielle Erbs-Hansen
- Min Kim
- Signe Hansen
- Nanna Larsson
- Trine Mølbæk
- Kasper Fischer-Rasmussen
- Alex Hemmingsen
- Eric Qing Zou
- Cristina Leal Rodríguez
- Kristina Aagaard
- Maria Hernandez Lorca
- Emil Høj Christiansen
- Thomas Rosenbom
- Iva Šunić
- Liang Chen
- Casper Sahl Poulsen
- Mina Ali
- Tingting Wang
- Ann-Sofie Colding Lund
- Nina Flensborg
- Jay Jiang
- Tamo Sultan
- Camilla Grube
- Michael Widdowson
- Alexandra Dalgas-Madsen
- Frederikke Østergaard
- Rikke Brandt Bangsgaard
- Johannes Wilm
- Parvaneh Ebrahimi
- Zhi Ye
- Zhuobing Peng
- Michael Forsmann
- Urvish Trivedi
- Beatriz Gomes
- Kathrine Jacaob
- Camille Ramm-Larsen
- Ulrikka Boulund
- Helena Hermansen
- Marie Louise Jahn
- Dorthe Klitmose Dahlstrøm
- Kaare Tranæs
- Amalie Klein-Petersen
- Frida Sidenius Jensen
- Lars Hjortshøj
- Jay Jiang
- Karen Hansen
- Location
- Funding
- Sponsors
- Contact
- Open positions
- Prægraduat Næstved
- Research Manager
- Prægraduat Næstved
- Forskningsassistent til klinisk børneforskning
- PhD opslag COPSAC
- Postdoc in early life human virome
- PhD opslag COPSAC
- Postdoc position in early life human virome
- PhD or Postdoc position within mechanisms and shared origin of immune mediated diseases
- PhD position in bioinformatics and human genetics
- IT – studenter hjælp til forskningscenter søges
- Forskningsassistent søges til klinisk børneforskning
- Metabolomics postdoc position
- Softwareudvikler / programmør
- PhD opslag COPSAC
- IT-udvikler og supporter med bredt kendskab til Django og Python
- Postdoctoral position at COPSAC
- Postdoctoral position at COPSAC
- Postdoctoral position at COPSAC
- Forskningsassistent til klinisk børneforskning
- Metabolomics research scientist
- Full-stack Python developer
- DXA skanningsuddannet til klinisk børneforskning
- Forskningsassistent til klinisk børneforskning
- PhD projekter indenfor Børneastma eller relaterede sygdomme
- Forskningsassistent til klinisk børneforskning
- Postdoc position in early life human virome
- PhD fellowship in computational life science
- Forskningsassistent til klinisk børneforskning
- Ph.d.-projekt omhandlende det tidlige miljøs betydning for børneeksem
- Junior Developer
- PhD projekt
- Kommunikationsmedarbejder med fokus på videnskabsformidling, hjemmeside og sociale medier
- Postdoc for Funding and Strategy
- PhD student to explore the acute airway microbiome in childhood asthma
- Forskningsassistent til klinisk børneforskning
- Organization Diagram
- Scientific visitors
- Research team (new look)
- Research Students
- Sponsors
- Expatriates
- Board of Directors
- Persondatapolitik
- COPSAC Alumni
- Research Students
- Hall of Fame
- COPSAC Alumni
- Research team
- Methods
- Dissemination
- Research Awards and Honors
- Publications
- Theses
- 2015 Ann-Marie Malby Schoos, MD PhD
- 2014 Marie Kragh, MSc PhD
- 2014 Eskil Kreiner-Møller, MD PhD
- 2014 Nadja Hawwa Vissing, MD PhD
- 2014 Anna Hammerich Thysen, Msc PhD
- 2013 Charlotte Giwercman Carson MD, PhD
- 2013 Anne Louise Bischoff MD, PhD
- 2012 Louise Pedersen, MD, PhD
- 2012 Jakob Stokholm, MD, PhD
- 2012 Nilofar Følsgaard, MD, PhD
- 2011 Martin Brasholt, MD, PhD
- 2011 Bo Chawes, MD, PhD
- 2010 Klaus Bønnelykke, MD, PhD
- 2010 Porntiva Poorisrisak, MD, PhD
- 2009 Mette N Hermansen, MD, PhD
- 2006 Liselotte B Halkjær, MD, PhD
- 2006 Birgitte Boysen Kjær, MD, PhD
- 2004 Lotte Loland, MD, PhD
- 2002 Frederik F Buchvald, MD, PhD
- 1999 Marianne Stubbe Østergaard, MD, PhD
- 1993 Jytte Fogh, MD, PhD
- 2017 Elín Bjarnadóttir, MD PhD
- 2017 Helene Wolsk, MD
- 2017 Tine Marie Pedersen, MD
- 2017 Astrid Sevelsted, MSc
- 2017 Rebecca Kofod Vinding, MD
- 2019 Lambang Arianto, MD
- 2018 Henrik Hallas, MD
- 2018 Jonathan Thorsen, MD
- 2018 Nadia Rahman Fink, MD
- 2019 Christian Carlsson, MD
- 2019 Christian Carlsson, MD
- 2019 Ni Wang, MD
- 2021 Sarah Nørgaard – MSc
- 2020 Asja Kunøe – MD
- 2021 Nicklas Brustad – MD
- 2021 Anders Eliasen – MSc
- 2021 Lærke Sass – MD
- 2022 Pia Nørrisgaard – MSc
- 2022 Emil Christensen – MD
- 2023 Rikke Sunde – MD
- 2023 Julie Kyvsgaard – MD
- 2024 Yang Luo – MSc
- 2024 Julie Rosenberg – MD
- 2024 Christina Poulsen – MSc
- 2024 Parisa Mohammadzadeh – MD
- 2024 Signe Jensen – MD
- 2024 David Horner – MD
- Literature for parents
- News
- DMSc theses
- Hall of Fame
- Newsletter
- Publications
- Research Strategy
- COPSAC cohorts
- COPSAC2000 cohort
- COPSAC2010 cohort
- Biobank Inventory
- COPSAC2010 Environmental exposures
- COPSAC2000 Environmental exposures
- COPSAC2010 Biobank
- COPSAC2000 Biobank
- COPSAC2000 Clinic
- COPSAC2010 Clinic
- COPSAC2010 Omics
- COPSAC2000 Omics
- COPSAC2000 Biomarkers
- COPSAC2010 Biomarkers
- COPSAC severe cohort
- COPSAC acute cohort
- Research Clusters
- Symposia
- ERC Grant Bo Chawes

Objective assessments
Physical Examination
A full physical examination is performed by the doctors employed at the COPSAC clinical research unit at every scheduled and acute visit.
Bone Mineral Density
Bone mineral density was assessed by ultrasound absorptiometry at the phalanx.
DEXA Scans
Dual energy x-ray absorptiometry (DEXA scans) was performed with GE Lunar iDXA objectively assessing body composition in terms of body fat, muscle mass, and bone mineral density/concentration.

Growth and Body Composition
Antropometrics
Length/height, weight, head- and abdominal circumference are measured at every scheduled visit at the COPSAC research unit. Weight was measured using calibrated digital weight scales, length was measured at 0-2 years of age by infantometer (Kiddimetre), thereafter, height was measured by Harpenden stapediometry. Growth curves are administered to the parents at the scheduled visits.
SYMPTOM RECORDINGS
Since birth, wheeze was recorded by the parents in daily diaries as composite dichotomized scores (yes/no).
Wheeze was explained as wheeze or whistling sounds, breathlessness or recurrent troublesome cough severely affecting the wellbeing of the child. In addition, the parents were given a book describing preschool wheezy symptoms.
The doctors at the COPSAC research unit reviewed symptom definition and the diary entries with the parents at the six-monthly clinical sessions and at acute clinic visits for wheezy episodes.

Ventilation tube insertions
Information on all ventilation tube insertions in the first 3 years of life were extracted from two national registries; The Danish National Patient Registry using the International Classification of Diseases 10th revision [ICD-10] code KDCA20 for all procedures performed at hospitals, and The Danish National Health Service Registry using procedure code 3009 for all procedures performed in private ear-nose-throat practices.
All information in the Danish registers are linked with a unique personal identification number (assigned by the Danish Civil Registration System to all people with permanent residency in Denmark), which made it possible to link the ventilation tube procedures to the children in our cohort.
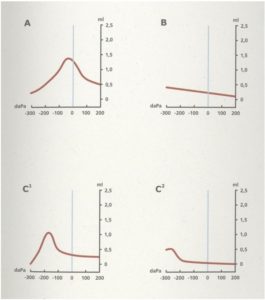
Tympanometry
Middle ear pressure is assessed by tympanometry on both ears. In a clear external auditory canal the tympanometric probe is inserted till an airtight seal is obtained. The probe changes the pressure in the external auditory canal, generates a pure tone and is hereby able to measure the compliance at the different pressure levels and assessing the middle ear pressure. This measurement is used to diagnose middle ear effusion.
Upper Airways
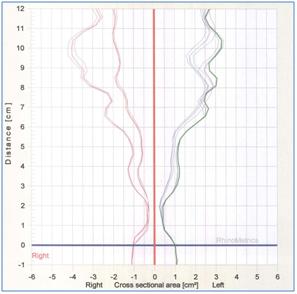
Acoustic Rhinometry
Upper airway patency is assessed by acoustic rhinometry with a RhinoMetrics SRE 2100 continuous wide-band acoustic rhinometer according to standardized international guidelines. The child is seated facing the examiner and stop breathing for about 5 seconds with the probe tube applied to the nostril. Three independent measurements with a standard deviation less than 5% are obtained from each nostril before and 15 minutes after decongestion from topical alpha-agonist.
The primary upper airway patency outcome is the decongested nasal airway volume 1-4cm from the probe at nares (VOL1-4).
Physical activity
Accelerometry
An omnidirectional accelerometer Actical (MiniMitter®, USA) assessing minute-by-minute activity counts was placed on the lateral side of the right or left ankle with a strap.
The parents and the child were instructed to leave it on day and night including during bathing. This specific monitor is especially suitable to capture the omnidirectional movements of young children. The outcome can be used both as a meassurement for daytime activity and as a meassurement for sleep quality. Studies have shown that children with asthma have a lower physical fitness level than their non-asthmatic peers.
Indoor Air Quality
Tobacco Smoke Exposure
Environmental tobacco smoke exposure is evaluated by cotinine and nicotine levels in samples of serum, urine and hair.
Child’s Bedroom Air
Fine-particle (<2.5µm) particular matter (PM2.5) concentration is measured using a cyclone in the child’s bedroom during 2 periods in the first year of life. Nitrogen oxide levels (NOx) are measured using Palmes tubes and volatile organic compounds (VOC) are measured using Radiello tubes in the bedroom for 10 weeks on 2 to 3 occasions during the first year of life. Indoor humidity and temperature are logged regularly during the autumn months using a HOBO H8 RH/Temp Logger.
Allergens in Bedding
The child’s bed and bedding is vacuumed during the first year of life and dust samples are analyzed for concentrations of cat and dog dander and levels of D. pteronyssinus og D. farinae.
Endotoxin Load
Endotoxin, a part of the cell-membrane of gram-negative bacteria, is believed to play a part in the gene-environmental interaction of atopic disease development. The component is airborne and can be identified in collected samples of house-dust. The families received a kit for home-sampling of house-dust from the child’s bed linen. With the kit came an adaptor, designed to connect dust-filter with vacuum-cleaner. The families were instructed to vacuum the child’s bed linen and mattress for 5 minutes respectively, loosen the filter and store the sample at -20°C for a minimum of 3 hours before returning it to COPSAC.
The families also received a kit for home-sampling of airborne dust. The families were instructed to place a cardboard tray (29x29x3cm) on a shelf in the child’s bedroom, approximately 2 m from the floor and minimum 20 cm from the roof. After 3 months, the tray was vacuumed with the same type of device as the bed-linen in the previous set-up. The cardboard tray was mailed to COPSAC.
Outdoor Air Quality
Daily air pollution levels for particulate matter <10µm (PM10), concentrations of ultrafine particles, nitrogen dioxide (NO2), nitrogen oxide (NOx) and carbon monoxide (CO) are available from a central background monitoring station in Copenhagen.
Diet
Maternal dietary intake during pregnancy
Information about maternal dietary intake during pregnancy was obtained from a food frequency questionnaire (FFQ) completed by the mothers in the 24th week of gestation. The FFQ was semiquantitative and consisted of 360 items of food and beverages covering the last 4 weeks of dietary intake.
The FFQ had three main components: food frequency, dietary supplements and other information.
Dietary intake at 1 year of age
Food frequency questionnaire filled out by the parents, contained questions about breastfeeding habits and length, time for introduction of solid food items and the children’s food consumption around 1 year after birth.
Breast milk
Mature post colostrum breast milk is collected one month after birth obtaining approximately 10 mL, which was subsequently aliquoted and stored at -80°C until analysis. The samples are analyzed for presence of 19 pro-inflammatory and immune regulatory cytokines and chemokines (TNF-α, IL-1β, IFN-γ, IL-4, IL-5, IL-10, IL-13, IL-17, IL-8, TGF-β1, IP-10, MCP-1, MIP-1β, MDC, GRO-α, Eotaxin-3, TARC, TSLP, and RANTES). Furthermore, the samples are analyzed for fatty acid composition, and we plan to perform analyses for dioxins, estrogen and end endocrine disrupters.
Lung function
Infant Spirometry
There are many ways of estimating neonatal lung function, but only infant spirometry provides volume anchored measurements, which can be reliably related to spirometry assessments later in childhood. We conducted infant spirometry by forced flow-volume measurements applying the Raised Volume Rapid Thoracic Compression Technique during chloral hydrate 90 mg/kg sedation in agreement with the American Thoracic Society (ATS) and European Respiratory Society (ERS) standards.
A non-expandable outer coat is wrapped around the infant’s chest and abdomen with an inflatable “balloon” inside. The infant’s lung volume is raised above the tidal range by inflating air through the pneumotachograph to a transrespiratory pressure of 2 kPa mimicking a full inspiration before a compression force is applied via the squeeze-jacket causing a full forced expiratory maneuver during which the flow was measured by a pneumotachograph with an aircushion facemask.The software identified the Forced Vital Capacity (FVC) as the first plateau on the volume-time curve; only measurements with FVC appearing after 0.5 second and with the Forced Expiratory Volume at 0.5 seconds (FEV0.5) being smaller than or equal to FVC were accepted.
Three to five acceptable curves were obtained at each measurement; the curve containing the median value of FEV0.5 was used for the analyses of both volume (FEV0.5) and flow (Forced Expiratory Flow at 50% of FVC; FEF50) parameters.
Spirometry
Forced expiratory volume in the first second (FEV1), forced vital capacity (FVC) and maximal mid-expiratory flow (MMEF) were assessed by flow-volume curves obtained by spirometry from up to five technically acceptable maneuvers in accordance with international criteria for reproducibility 4 using a MasterScope system spirometer (Erich Jäeger, Würtzburg, Germany). Spirometry was conducted at every scheduled and acute visit to the COPSAC research clinic initiated at 5 years of age. Bronchial reversibility to inhaled short acting ß2-agonist (bronchodilator response) was obtained with a computer-animated volume driven incentive well-known to the children.
Multiple Breath Wash-out (MBW)
Ventilation inhomogeneity was assessed by MBW with two different methodologies/tracer gasses:
MBW utilizing nitrogen (N2) as inert tracer gas was conducted with Exhalyzer D, Eco Medic software 3.1.3 (Eco Medics AG, Dürnten, Switzerland). Ambient settings, including room temperature, altitude, relative humidity and barometer pressure, were updated daily along with daily flow and volume calibration. Oxygen channel calibration was completed every week. The post-capillary dead space reducer setting was depended on their body weight (DRS 2: body weight 15-34.9 kg, DRS 3: body weight > 35 kg). During the wash-out, the children inhaled 100% oxygen delivered by a bypass flow at 1L/s until N2 end-tidal concentration of three consecutive exhalations were below 1/40th of the starting concentration, which represents the lung clearance index (LCI).
The software also provided information about dysfunction in the conductive airways (S-cond) and the acinary airways (S-acin) by evaluation of the phase III slope during the wash-out.
MBW utilizing sulfur hexafluoride (SF6) as inert tracer gas was done with the Innocor model Inn00400, software 7.00 (Innovision ApS, Glamsbjerg, Denmark). After the SF6 wash-in phase, the children completed the wash-out phase while watching a movie, wearing a nose-clip and breathing through a mouthpiece with a bacterial filter (DARTM Electrostatic Filter Small, Covidien). The software provided LCI, which but not S-cond or S-acin.
Each child completed at least three tests of both MBW methods. All tests were subsequently quality controlled for leaks, airflow and respiratory pattern, excluding tests with an unsteady wash-out or tidal breathing phase.
Whole Body Plethysmography
COPSAC has contributed substantially to develop whole-body plethysmography for measurement of lung function in preschool children from 2 years of age.
Principle
Specific airway resistance (sRaw) is measured as the ratio between the pressure (P) generated by the thoracic and abdominal excortions during tidal breathing and the resulting air flow (V’). sRaw is determined in a whole-body plethysmograph, where a transducer measures pressure changes in the sealed box and a pneumotachograph simultaneously measures the flow swing at the mouth. Flow and volume measurements are corrected to body temperature and pressure, saturated with water vapour (BTPS) conditions.
The software calculates sRaw as (delta P/ delta V’) x (Pamb – PH2O).
Equipment
MasterScreen Body Unit. (E. JAEGER GmbH, Wuerzburg, Germany), which is calibrated once daily.
We recommend a facemask with a large cushion, which ensures a good seal and stabilizes the cheeks and chin. A built-in flexible tube ensures that the mouth remains open to avoid nasal breathing.
Procedure
- The child is seated alone in the box with the door closed. The child’s breathing should aim for a frequency of 30-45.
- An adult may accompany the child in the box and should then perform one slow exhalation during the measurement. Such slow exhalation will not interfere in the measurement of the rapid pressure swings by the child, but sRaw should be adjusted by the factor (810-adults weight)/810).
- “Loops” on the screen shows the relation between pressure (or volume) (x-axis) and flow (y-axis). That is the pressure driving the flow in and out of the lungs. sRaw is the inclination of these loops.
- Technically acceptable loops are chosen as those which are “closed” in the middle “Open” loops normally indicate insufficient BTPS correction. The loops should assume a straight line with a tendency to s-shaped, and should be symmetric around the inclination.
- sRaw from one run is calculated as the median value of five technically satisfactory loops with similar configuration and inclination.
- It is recommended to calculate the mean sRaw of duplicate measurements. Such two measurements should not differ more than + 0,3 [kPa*s] sRaw loops before and after provocation in a young child with asthma.
Bronchial Challenge Tests
Methacholine Challenge in Neonates
At age one month, baseline FEV0.5 was measured by infant spirometry (see methods) after a saline inhalation and following subsequent inhalations of methacholine in quadrupling dose steps from 0.04 to 16.67μmol/L delivered by a dosimeter attached to a nebulizer (SPIRA 08 TSM 133; Respiratory Care Center; Hämeenlinna, Finland) (17,18). Bronchial reactivity was assessed by repeated measurements of FEV0.5 and transcutaneous oxygen pressure (PtcO2) (TCM3; Radiometer, Copenhagen, Denmark).
Bronchial reactivity to methacholine was subsequently estimated from the dose-response curves fitted with a logistic function as the provocative dose of methacholine producing a 15% decrease in PtcO2 (PD15) from baseline.
Methacholine Challenge in School-Aged Children
Methacholine challenge is performed at 6½ and 13 years of age in accordance with ATS recommendations. Initially, maximum baseline forced expiratory volume in the first second (FEV1) is assessed by spirometry; a FEV<65% of predicted leads to cancellation of the test.
If FEV1 is above 65% predicted, methacholine chloride is given with a dosimeter attached to a nebulizer (SPIRA 08 TSM 133; Respiratory Care Center; Hämeenlinna, Finland) in quadrupling dose steps from 0.06 to 14.4 µmol keeping inhalation flow <0,5 l/s. The test is completed when FEV1 has decreased 20% or the maximum dose is reached.
Bronchial responsiveness to methacholine is determined as the provocative dose of methacholine leading to a 20% fall in FEV1 (PD20) from baseline.
Cold Dry Air Challenge
Cold dry air challenge is performed by hyperventilating -18°C cold dry air. The air is generated by a Jäeger Respiratory Heat Exchange System. The test is done as a single-step isocapnic hyperventilation test lasting 4 minutes. An animated computer program guides the child to maintain an adequate frequency of breathing aiming at 1 l/min/kg body weight (see methods). A face mask fitted with a mouthpiece is used during hyperventilation ensuring mouth breathing and preventing inhalation of room air.
Exercise Challenge
Exercise challenge is performed in accordance with ATS guidelines. The child exercises 6 minutes on a motor driven QUINTON® CR60 treadmill with 10 degree steepness and adjustable speed. The speed progressively advances the first 1- 2 minutes until heart rate is 80-90% of predicted maximum. During exercise, the child breathes dehumidified atmospheric air through a face mask (Hans Rudolph; Kansas City, MO) preventing nasal respiration. Lung function is measured by spirometry before and 1, 3, 5, 10 and 15 minutes after exercise using the max percentage decline in FEV1 from baseline to the minimum value within 10 minutes after exercise as outcome.
FeNO in neonates
Concentration of FeNO was measured by an off-line technique, after the completion of infant spirometry. A mask covering the mouth and nose attached to a 2-way valve was gently placed on the infants face during sedation. A respiratory resistance of at least 5 cm H2O was achieved by a resistor fitted into the expiratory side of the valve. The infant inhaled ambient air, but measurements were cancelled if ambient NO exceeded 10 parts per billion (ppb). When stable tidal breathing was assured, an impermeable bag (Quintron Instrument©, Milwaukee, USA) was attached to the expiratory side of the valve and 750 ml mixed expired air was collected. Within 15 minutes, the bags were attached to the inlet of the NO analyzer and air was continuously sampled from the bags with a flow of 110 ml/min. Concentration of FeNO was measured using a chemiluminescence analyzer (EcoPhysics CLD 77 AM, Duernten, Switzerland). The sensitivity was 0.1 ppb and rise time (0-90%) was 0.1 second. The analyzer was calibrated at least once daily using certified NO gas (100 ppb) (AGA, Gothenburg, Sweden). FeNO levels were calculated as the mean of duplicate measurements in each infant.
FeNO in School-Aged Children
FeNO is measured by an online technique 7, 8 in accordance with ATS guidelines at all scheduled and acute visits initiated at age 5 year by either Niox (Aerocrine Nitric Oxide Monitoring system 02100 NO Gas Analyzer) or Niox Mino (Aerocrine).
The child is comfortably seated and breaths quietly for about 5 minutes to acclimatize. Thereafter, the child inhales to near total lung capacity and immediately exhales at a constant flow of 50 ml/s until a FeNO plateau of 2 seconds is identified. An exhalation last at least 4 seconds and the expiratory pressure is maintained at 5-20 cm H2O to close the velum. During exhalation, the child is guided by an exhalation flow driven animated computer program.
Two repeated exhalations that agree within 5% are completed with 30 seconds intervals and mean FeNO is recorded.
Exercise challenge
Exercise induced bronchoconstriction (EIB) is a hallmark of asthma and particularly it is a key-symptom in pediatric asthma. EIB reflects uncontrolled disease, but a history of EIB in children is of limited accuracy and subject to recall bias by parents. EIB testing is therefore useful to diagnose and monitor asthma in children and has a fair sensitivity, specificity and repeatability.
Hyperventilation test
COPSAC has developed a computer program assisting the child to achieve the correct breathing frequency during hyperventilation with dry or cold air. The method is particularly useful in young children.
Microbiology
Vaginal Flora
Vaginal swabs are collected from asymptomatic pregnant women at gestational week 24 and 36 from fornix posterior of the vagina. Vaginal samples at pregnancy week 36 were characterized by conventional culture on selective and non-selective media within 24 hours after collection. The remaining part of the sample is stored at -80º C for future analysis. All bacteria identifications are confirmed biochemically by automated identification system VITEK 2 (Bio Mérieux, France).The microbiome at pregnancy week 24 and 36 has furthermore been characterized using culture-independent ribosomal RNA (rRNA) sequencing of the 16S ribosomal subunit’s hypervariable V4 region.
Fecal Bacteria
Fecal samples were collected at age 1 month and 1 year. Conventional cultures were made with identification of aerobic, anaerobic and yeast/fungi species. Additionally, the V3 region of bacterial 16S rRNA was isolated for PCR-Denaturing Gradient Gel Electrophoresis (DGGE). DGGE is based on the principle that increasing denaturant concentration will melt double-stranded DNA in distinct domains. When the melting temperature of the lowest domain is reached, the DNA will partially melt, creating branched molecules with reduced mobility in a polyacrylamide gel visible as single size bands. The fecal microbiome has furthermore been characterized for all time points using culture-independent ribosomal RNA (rRNA) sequencing of the 16S ribosomal subunit’s hypervariable V4 region.
Skin Bacteria
The children were investigated for colonization with Staphylococcus aureus (including antibiotic susceptibility and phage typing) and Streptococcus species in vestibulum nasi and perineum. In addition, swabs were performed from involved skin at acute visits due to skin problems.
Skin swabs have been collected for 16S rRNA gene amplicon sequencing.
Airway Microbiology
Hypopharyngeal sampling was performed in asymptomatic infants at the scheduled visits with a soft suction catheter passed through the nose. No antibiotics were prescribed 14 days prior to sampling and only aspirates including columnar epithelial cells and/or mucus with leucocytes were eligible.
- Bacteria samples were cultured with the use of standard methods for Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus aureus and Streptococcus pyogenes. The airway microbiome has furthermore been characterized using culture-independent ribosomal RNA (rRNA) sequencing of the 16S ribosomal subunit’s hypervariable V4 region.
- Virus samples were analyzed by PCR for Rhinoviruses, enteroviruses, RSV types A & B, influenza virus types A H1, A H3 and B, parainfluenza viruses, coronaviruses, adenoviruses, and human metapneumoviruses.
- Acute samples were at obtained at day 3 of every respiratory exacerbation during the first 3 years of life. Samples were cultured for bacteria and viruses were identified using PCR.
Throat swabs have been collected for 16S rRNA gene amplicon sequencing.

Genetics
Candidate Gene/SNP Approach
DNA is purified from blood samples of children and parents and stored at -80°C for later genotyping.
Genome-Wide Genotyping
High throughput genome-wide single-nucleotide polymorphism (SNP) genotyping is performed in the COPSAC trios (children and parents), using the IlluminaInfinium™ II HumanHap 550K SNP BeadChip technology (Illumina, San Diego) and further by the Illumina Infinium™ HumanOmniExpressExome BeadChip in the children.
mRNA Expression Data
Local airway epithelium biopsies are collected by scraping the nasal cavity laterally posterior of the anterior portion of the inferior turbinate with Rhinoprobes.The material is stabilized in QIAGEN RNAprotect Cell Reagent. Following, mRNA is purified, chipped, and analyzed.
Psychological evaluation
Bailey
Bayley Scales of Infant and Toddler Development®, Third Edition (Bayley-III®)
The Bayley test assesses infant and toddler development across five domains; Cognitive, Language, Motor, Socio-Emotional and Adaptive. We only use the Cognitive part of the test. The test is an individually administered instrument where interactive play with the child is used to assess sensorimotor development, exploration and manipulation, object relatedness, concept formation, memory and other aspects of cognitive processing.
Sensitization
Specific IgE measures in Plasma and Serum
Blood samples are collected and measured for specific-IgE and total-IgE levels are determined using a screening method (ImmunoCAP, Phadiatop Infant™, Pharmacia Diagnostics AB, Uppsala, Sweden). A blood sample is further analyzed using ImmunoCAP ISAC® measuring 112 components from 51 different allergen sources. Levels ≥ 0.3 ISAC Standardised Units (ISU) is considered indicative of allergic sensitization. The 18 months sample is further analyzed for specific-IgE to dog, cat, egg, and milk using ImmunoCAP, and any level ≥ 0.35 kUA/L is considered indicative of sensitization.
Skin Prick Test
Skin prick test (SPT) is performed with ALK-Abelló Soluprick SQ. SPT is performed using standard allergen extracts (ALK Abello, Soluprick® SQ) of 10 inhalant allergens (dog, cat, horse, birch, timothy grass, mugwort, D. pteronyssinus, D. farinae, Cladosporium herbarum and Alternaria alternata), 10 food allergens (milk, egg, wheat flour, rye flour, soybean, cod, peanut, oatmeal, pork and beef), and with fresh cow’s milk and pasteurized hen’s egg. Histamine dihydrochloride (10mg/mL) and physiological sodium chloride (9mg/mL) is used as positive and negative controls, respectively. Droplets of allergen extracts, fresh foods and controls are applied to the child’s volar forearm and a sterile lancet is used to prick through the droplets approximately 1mm through the skin barrier. The reaction to the positive control is read after 10min while the reactions to the allergens and negative control are read after 15min. A positive reaction is defined as a wheal diameter ≥2mm larger than the negative control at age <1½ years and ≥3mm at age >1½.
Plasma and Serum
Serum sIgE levels is determined by a screening method (ImmunoCAP, Phadiatop Infant™, Pharmacia Diagnostics AB, Uppsala, Sweden) against the same panel of inhalant and food allergens as listed above for SPT including Penicillium notarum, Aspergillus fumigatus, shrimp, potato, hazelnut, brazil nut, almond and coconut. A sIgE level ≥0.35kUA/L for a specific allergen is considered indicative of allergic sensitization.
Mononuclear Cells
Mononuclear cells are isolated and stored in liquid nitrogen at -150°C.
Inflammatory Markers
Nasal Eosinophils
Nasal mucosal specimens were obtained by gently scraping the anterior part of the inferior turbinate with Arlington Rhinoprobes. The specimens were transferred onto glass slides, air-dried for 30 minutes, fixed in 95% ethyl alcohol for 3 minutes and stained by May-Grünwald-Giemsa method. Eosinophils were counted by light microscopy at high-power by two experienced cytologists. Rating was done according to Meltzer’s semi-quantitative scale.
Eosinophil.
Urinary Markers
At <3 year of age urine was collected as a spot sample into a sterile plastic bag adherent to the skin.
Eosinophil protein-X concentrations are determined by a double-antibody RIA immunoassay at ages 1 and 6 month. Cysteinyl leukotriene and prostaglandin D2 concentrations are measured by Neogen ELISA test kits at age 1 month. To standardize for variations in renal excretion, all urinary markers are adjusted for creatinine excretion. Urinary creatinine level is measured by buffered kinetic Jaffé reaction.
Blood Inflammatory Markers, cord blood
Midwives received written instructions on the collection of cord blood by needle puncture from the umbilical cord vein: 14 ml cord blood was collected (7 ml in an EDTA tube and 7 ml whole blood) and subsequently send to the COPSAC research unit. Levels of the chemokines CXCL10, CXCL11, CCL17 and CCL22 were analysed with an in-house multiplexed Luminex assay.
Blood Inflammatory Markers, blood
High sensitivity C-reactive protein (hs-CRP), interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α) and chemokine (C-X-C motif) ligand 8 (CXCL8) were measured in plasma. Levels of inflammatory biomarkers were determined by high-sensitivity ELISA assays based on electro-chemiluminescence in a 4-plex setting for IL-1β, IL-6, CXCL8 and TNF-α and as a single high-sensitivity assay from MesoScale Discovery for hs-CRP. Samples were read in duplicates with the Sector Imager 6000 (MesoScale Discovery®, Gaithersburg, MD, USA). Blood eosinophil count was determined.
Breathomics
Volatile Organic Compounds (VOC) in exhaled breath is proposed as a non-invasive marker of airway inflammation. Exhaled breath for VOC analysis is sampled in the ABC children at every planned visit and at every episode of acute lower respiratory symptoms. Breath samples are collected in impermeable 750 ml bags of chemically inert material, connected to a facial mask covering the mouth and nose by a double one-way-valve (see figure). Breath samples are analyzed by an electronic nose (E-nose) and Gas Chromatography Mass Spectrometry technique (GC-MS).
E-Nose
An Electronic Nose (Cyranose 320) is used to analyze patterns of VOCs in samples of exhaled air. The analytical system of the Cyranose 320 is based on a 32-carbon sensor array; a Nose-chip; reacting with the organic volatiles in the presented samples. The sensors react by absorbing and expanding, and the result is a difference in electronic resistance through the Nose-chip resulting in a smell print for each sample.
Gas Chromatography Mass Spectrometry
The expired air of the ABC infants is emptied from the sample bags into a stainless-steel two-bed sorption tube, filled with carbograph and stored at room temperature (see figure). The tubes are subsequently shipped to The Netherlands where our collaborator Prof. F.J.van Schooten, Department of Health Risk Analysis and Toxicology, Nutrition and Toxicology Research Institute Maastricht, Maastricht University, analyze and quantify the VOCs by thermal desorption-gas chromatography time-of-flight mass spectrometry (GC-MS) leading to quantification of overall more than 2000 VOC in each sample.
Blood Inflammatory Markers
High sensitivity C-reactive protein (hs-CRP), interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α) and chemokine (C-X-C motif) ligand 8 (CXCL8) were measured in plasma at age ½ year. Levels of inflammatory biomarkers were determined by high-sensitivity ELISA assays based on electro-chemiluminescence in a 4-plex setting for IL-1β, IL-6, CXCL8 and TNF-α and as a single high-sensitivity assay from MesoScale Discovery for hs-CRP. Samples were read in duplicates with the Sector Imager 6000 (MesoScale Discovery®, Gaithersburg, MD, USA).
Mucosal lining fluid sampling
This is a non-invasive technique for sampling of undisturbed mucosal lining fluid from the upper airways, by which quantification of in situ levels of protein mediators such as cytokines and chemokines can be performed in subjects of all ages.
The mucosal lining fluid is sampled on a strips of filter paper (fibrous hydroxylatedpolyester sheets from Accuwik Ultra (cat no. SPR0730, Pall Life Sciences, Portsmouth, Hampshire, UK). One filter paper is inserted in each nostril, placed at the anterior part of the inferior turbinate, and left for 2 min of absorption. Analytes are eluted from the filter papers after addition of identical volumes of buffer to all samples. Thereafter, the extracted protein-based eluates are analyzed by an electro-chemoluminescence-
We have measured in situ levels of 20 pre-selected immune mediators related to specific immune signaling pathways in the upper airway mucosa, but the technique is not limited to that specific panel or sampling site.
This technique enables in situ quantification of the airway mucosal immune profile from birth and can be applied longitudinally, which has important applications for studying the effect of genetics and early life environmental exposures, the pathophysiology, endotyping and monitoring of respiratory diseases and for developing and evaluating novel therapeutics.






