Childhood asthma is often preceded by recurrent episodes of asthma-like symptoms during the first years of life. Severe episodes with asthma-like symptoms cause a high rate of emergency department (ED) visits and often require hospitalization with high socioeconomic demands. Typical triggers for asthma-like symptoms are respiratory tract infections (RTIs) of both viral and bacterial etiology, exercise, allergens, pollutants, and tobacco smoke. Exacerbations in preschoolers are most often triggered by RTIs and have a poor response to oral corticosteroids (OCS) that are one of few options for treating exacerbations in this age group. Preventivetreatment of asthma in preschoolers is maintenance inhaled corticosteroids (ICS) as monotherapy and/or an oral leukotriene receptor antagonist (LTRA), which is effective for achieving daily symptom control, whereas many children still experience exacerbations requiring OCS and/or hospitalization. Currently, there are no interventions aiming at reducing the high morbidity associated with acute exacerbations, which is a major unmet clinical need in this age group of vulnerable young children.
Previous randomized controlled trials (RCTs) suggest antibiotics for treating episodes of asthma-like symptoms in preschool children. Further, high-dose vitamin D supplementation has been shown to reduce the rate of asthma exacerbations among adults with asthma, while RCTs in preschool children are lacking. The aims of this combined RCT are to evaluate treatment effect of azithromycin on episode duration and the preventive effect of high-dose vitamin D supplementation on subsequent episodes of asthma-like symptoms among hospitalized preschoolers.
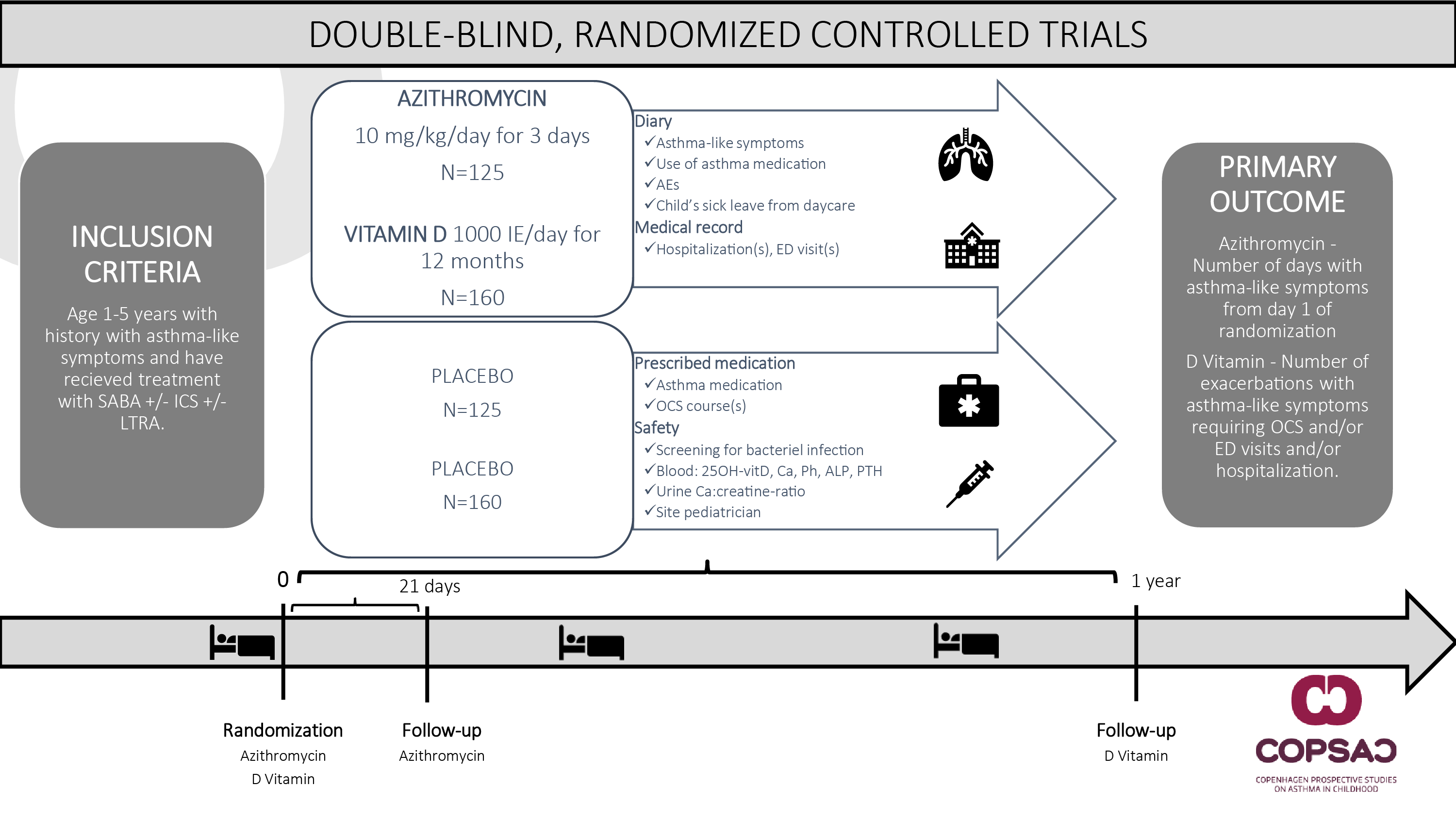
Project Outline
Eligible participants are 1–5-year-old children with a history of recurrent asthma-like symptoms, hospitalized due to an acute episode will be randomly allocated 1:1 to azithromycin (10 mg/kg/day) or placebo for 3 days (n=250). Further, independent of the azithromycin intervention, participants will be randomly allocated 1:1 to high-dose vitamin D (1000 IU/day + standard dose 400 IU/day) or standard dose (400 IU/day) for 1 year (n=320). Participants are monitored with electronic diaries for asthma-like symptoms, asthma medication, adverse events, and sick-leave. The primary outcome for the azithromycin intervention is duration of asthma-like symptoms after treatment. Secondary outcomes include duration of hospitalization and anti-asthmatic treatment. The primary outcome for the vitamin D intervention is the number of exacerbations during the treatment period. Secondary outcomes include time to first exacerbation, symptom burden, asthma medication, and safety.
Trial registration number
https://clinicaltrials.gov, NCT05028153, NCT05043116.
https://eudract.ema.eurupa.eu, 2020-004420-42.
Danish local ethical committee, H-20065249, H-20066827.